Filters
Done-

 58.30€
58.30€20 mg/ml crosslinked hyaluronic acid injectable
INDICATION
PERFECTHA® – DERM is indicated for the filling of superficial skin depressions, to reduce peri-orbital and peri-buccal fine lines as well as those on the forehead.
PACKAGING
1 x 1 ml
2 x 30G needlesPlease read package leaflet before use.
-

 156.20€
156.20€20 mg/ml crosslinked hyaluronic acid injectable
INDICATION
PERFECTHA® – SUBSKIN is indicated for redefining contours or giving volume to malar areas, cheeks and chin projection or in more serious cases, for facial atrophy, to remodel the contour of the hands and the bridge of the nose, and in the treatment of sub-cutaneous depressions and scars.
PACKAGING
3 x 1 ml
1 x 21G cannulaPlease read package leaflet before use.
-

 109.20€
109.20€Semi-solid calcium hydroxyapatite injectable
INDICATION
The device is indicated for plastic and reconstructive surgery, including deep dermal and sub-dermal soft tissue augmentation of the facial area, and is also intended for restoration and/or correction of the signs of facial fat loss (lipoatrophy) in people with human immunodeficiency virus.
PACKAGING
1 x 1.5 ml
Please read package leaflet before use.
-

 111.60€
111.60€Semi-solid calcium hydroxyapatite injectable with 0.3% lidocaine
INDICATION
The device is indicated for plstic/reconstructive procedures, including deep dermal and subdermal soft tissue augmentation of the facial area and is also intended for restoration and correction of facial volume loss.
PACKAGING
1 x 1.5 ml
2 x 27G needlesPlease read package leaflet before use.
-

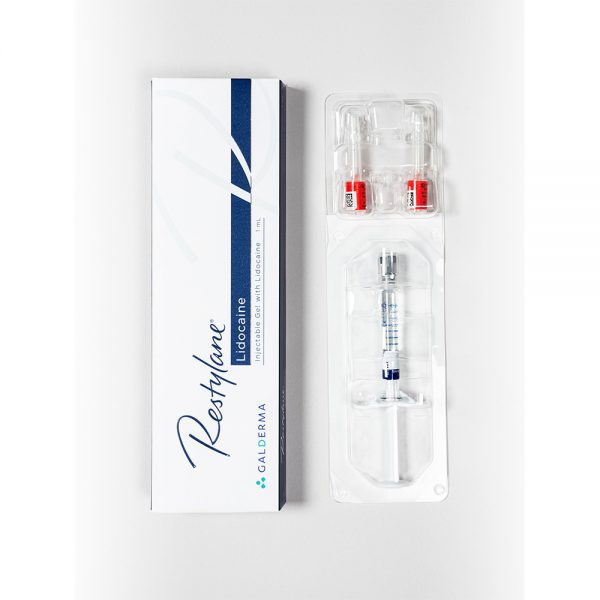 74.00€
74.00€Restylane Lidocaine is a sterile, transparent, biodegradable gel of stabilized hyaluronic acid of non-animal origin with the addition of 0.3% lidocaine hydrochloride. It is supplied in a glass syringe. The contents of the syringe are sterilized using moist heat. The product is for single use only. Disposable 29G TW (thin-walled) needles sterilized using ethylene oxide are provided. To ensure traceability the patient record label (part of the syringe label) schould be attached to patient records.
INDICATION/INTENDED USE
This product is intended to be used for facial tissue augmentation. It is recommended to be used for shaping the contours of the face, the correction of folds and for lip enhancement. It should be injected into the middle part of the dermis or in the submucosal layer of the lip. For facial areas with limited soft tissue support and soft tissue cover,e.g. the periorbital region, injection into the subcutaneous fatty tissue or supraperiostal administration are recommended. The addition of lidocaine provides a pain relieving effect during treatment.
PACKAGING
1 x 1 ml
2 x 29G needlesPlease read package leaflet before use.
-

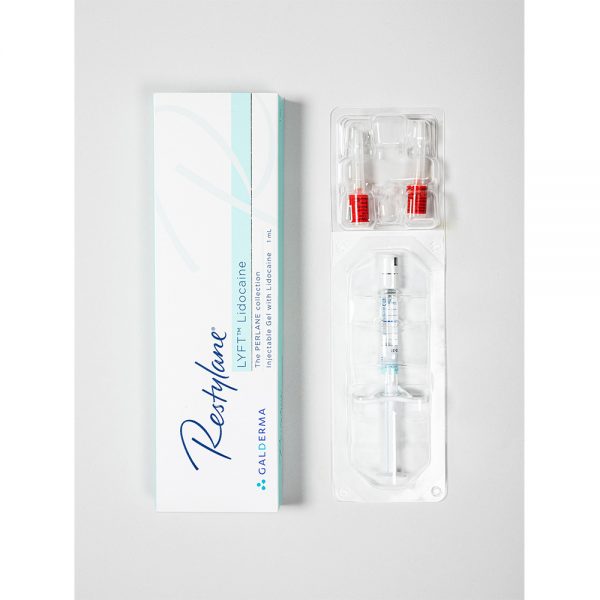 82.80€
82.80€Restylane Lyft Lidocaine is a sterile, transparent, biodegradable gel of stabilized hyaluronic acid of non-animal origin with the addition of 0.3% lidocaine hydrochloride. It is supplied in a glass syringe. The contents of the syringe are sterilized using moist heat. The product is for single use only. Disposable 29G TW (thin-walled) needles sterilized using ethylene oxide are provided. To ensure traceability the patient record label (part of the syringe label) schould be attached to patient records.
INDICATION/INTENDED USE
This product is intended to be used for facial tissue augmentation. It is recommended to be used for shaping the contours of the face, the correction of folds and for lip enhancement. It should be injected into the deep layer of the dermis and/or the surface layer of the subcutis or in the submucosal level of the lip. For facial areas with limited soft tissue support and soft tissue cover,e.g. the periorbital region, injection into the subcutaneous fatty tissue or supraperiostal administration are recommended. The addition of lidocaine provides a pain relieving effect during treatment.
PACKAGING
1 x 1 ml
2 x 29G needlesPlease read package leaflet before use.
-

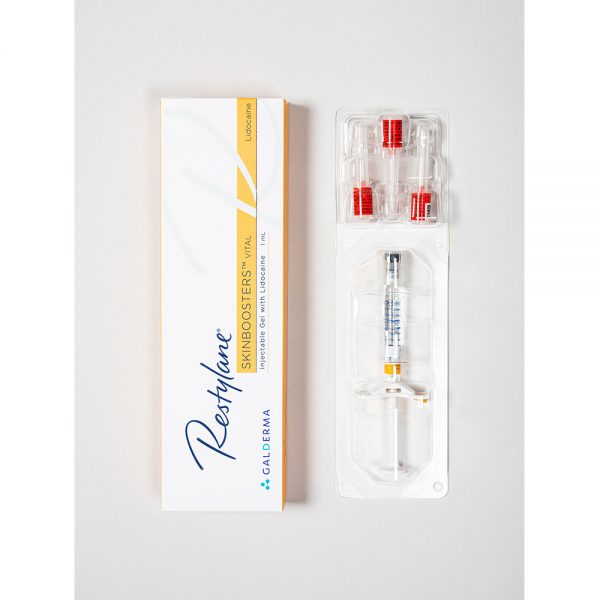 64.40€
64.40€Restylane Skinboosters Vital Lidocaine is a sterile, transparent gel of stabilized hyaluronic acid of non-animal origin with the addition of 0.3% lidocaine hydrochloride. It is supplied in a glass syringe. The product has a built in dose-guide, Smart Click System, which when activated creates a clicking sound to indicate each injected dose. The 1 ml syringe gives approximately 100 doeses. The contents of the syringe are sterilized using moist heat. The product is for single use only. Disposable 29G TW (thin-walled) needles sterilized using ethylene oxide are provided. To ensure traceability the patient record label (part of the syringe label) schould be attached to patient records.
INDICATION
This product is intended to restore skin hydrobalance, improve skin structure and the elasticity of the skin. It should be injected in the dermal layer of the skin, preferably in the deeper part of dermis. The addition of lidocaine provides increased overall treatment comfort.
PACKAGING
1 x 1 ml
3 x 29G needlesPlease read package leaflet before use.


